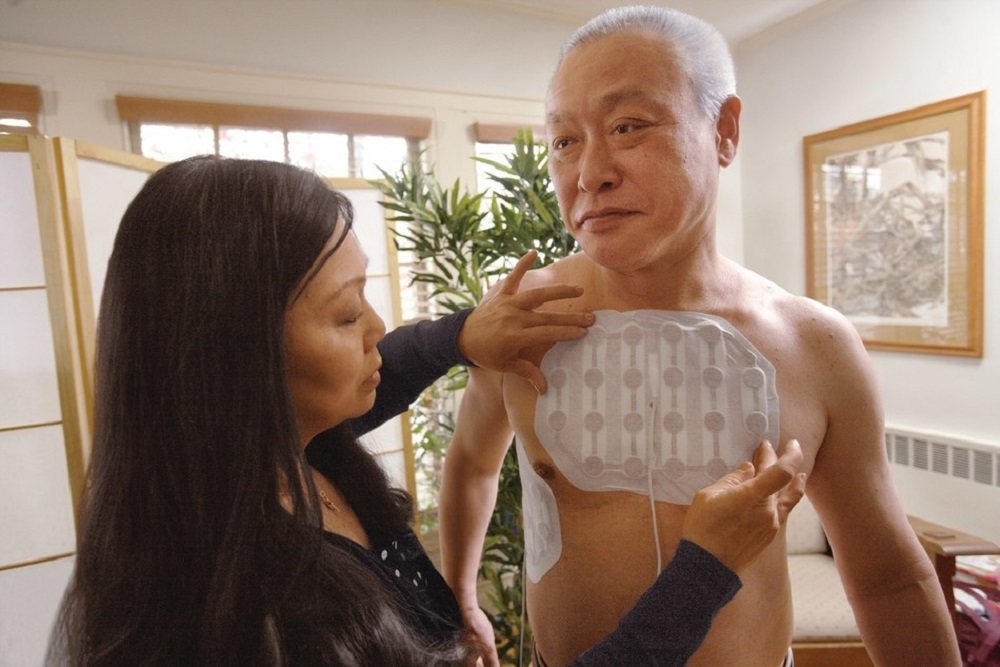
WEST CANCER CENTER: The first center in the country to offer the first FDA-approved Mesothelioma treatment in more than 15 years
A clinical study showed that the NovoTTF-100L™ System in combination with chemotherapy may help people with malignant pleural mesothelioma extend their lives